Blog Post
Quality Standards in Sports Nutrition
Quality standards such as AJI97, BP, EP, and USP play a crucial role in ensuring the safety, efficacy, and quality of sports nutrition. These standards provide specifications for the identity, purity, strength, and quality of substances used in the production of pharmaceuticals and other goods, such as Sports Nutrition. This information about the quality standards and compliance with specific pharmacopoeias can often be found in the product's specifications or documentation. But what do these quality standards mean?
QUALITY STANDARDS
Different Quality Standards
AJI97 (Japanese Pharmacopoeia)

Origin: AJI standards are established by Ajinomoto, a Japanese company known for producing amino acids. AJI97, for example, refers to the 1997 edition of AJI standards.

Application: AJI standards are primarily applied to amino acids, ensuring the quality and purity of these compounds. They are used in pharmaceuticals, food, and various other applications where amino acids play a crucial role.

Focus: The primary focus of AJI97 and other AJI standards is to set quality specifications for amino acids, supporting their safe and consistent use in different industries.
BP (British Pharmacopoeia)

Origin: The British Pharmacopoeia (BP) is the official pharmacopoeia of the United Kingdom.

Application: The BP provides standards for the quality and purity of pharmaceutical substances, excipients, and medicinal products. It is widely used in the UK and other countries that follow British standards.

Focus: BP focuses on ensuring the quality, safety, and efficacy of medicines through specifications for their ingredients and formulations.
EP (European Pharmacopoeia)

Origin: The European Pharmacopoeia (EP) is a product of the European Directorate for the Quality of Medicines & HealthCare (EDQM).

Application: EP is used to harmonize standards for the quality of pharmaceuticals and their components across European countries

Focus: EP focuses on ensuring the quality, safety, and efficacy of medicines, providing monographs for active substances, excipients, and finished products.
USP (United States Pharmacopeia)

Origin: The United States Pharmacopeia (USP) is a compendium of drug information and standards used in the United States.

Application: USP standards are used for drugs, dietary supplements, and other healthcare products in the United States.

Focus: USP focuses on setting standards for the identity, strength, quality, and purity of drugs and related products to ensure their safety and efficacy.
HOW TO CHOOSE
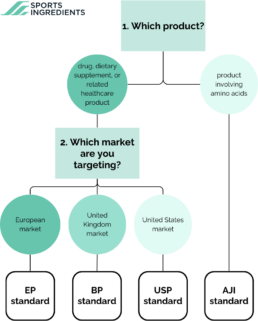
When to choose which Quality Standard?
1. Which product? When it is about products that involve amino acids, we mostly use AJI97. AJI standards are specific to amino acids and are often used in Japan and other regions where these standards are recognized. Relevant for products involving amino acids and used in markets where AJI standards are recognized. When it is about a drug, dietary supplement, or related healthcare product, we can use EP, USP or BP.
2. Which market are you targeting?
European Market: Choose EP if you are targeting the European market. Compliance with EP standards is often required for pharmaceutical products seeking marketing authorization in European Union member states. If your product needs to comply with European Union regulatory requirements, EP is essential. United States: Opt for USP if your target market is the United States. USP standards are widely recognized and used for regulatory compliance in the U.S.. Compliance with USP standards is crucial for obtaining regulatory approval in the United States. United Kingdom: If you are focusing on the United Kingdom or other regions that follow British standards, BP is the appropriate choice. Appropriate for products seeking approval in the United Kingdom and other regions following British standards.
We advise on Quality Standards in Sports Nutrition
The quality standards are developed and regularly updated by respective pharmacopoeial organizations, and adherence to these standards is often required by regulatory authorities for the approval and marketing of pharmaceuticals and related products. Manufacturers in the pharmaceutical and related industries use these standards to ensure the consistency and quality of their products.
Related Products
How to read extract ratios?
Misunderstandings on what plant-to-extract ratios signify are common. These ratios are often thought to indicate the amount of active ingredient present or the purity of the extract. These are both common misinterpretations of plant-to-extract ratio’s. This blog post will tell you more about what these plant-to-extract ratios actually mean.
Protein content determination: is it always accurate?
Products containing protein are required to list the protein content on their nutritional labels. The determination and calculation of this protein content are commonly performed using the Kjeldahl method. This method measures the amount of nitrogen in the product to estimate its protein content. This blog will explain the Kjeldahl method and assess its accuracy.
Gastro-resistance explained
Nowadays dietary supplements are occasionally labelled gastro-resistant. Often the question arises what does gastro-resistant mean and what is it good for? This blog will give more insight into gastro-resistant supplements and their benefits.
Private Label Clear Vegan Protein
We are happy to unveil our latest product innovation – Clear Vegan Protein, designed to meet the evolving demands of the health and wellness market. This formula offers a refreshing and customizable alternative to traditional plant-based proteins, making it an ideal addition to your product lineup. Need help to kickstart your next Private Label Clear Vegan Protein? Let us help you to produce it.
Sweeteners for Sports Nutrition
Nowadays a lot of different non-nutritive sweeteners are available like sucralose, stevia, acesulfame K, and aspartame. As a manufacturer of sports nutrition it is important to be well-informed on which sweetener to use.
Labelling Requirements for Sports Nutrition
In the fast-paced world of sports nutrition, where performance and precision are paramount, it is critical to understand the importance of regulatory compliance and transparency. Whether you're a manufacturer, distributor, or retailer of sports nutrition products, adhering to precise labelling requirements is not only a legal obligation but also a key component of maintaining the trust and loyalty of your customers.
Heat Stability of Proteins in Sports Nutrition
Proteins, the building blocks of life, undergo a fascinating transformation when exposed to heat. As a manufacturer, understanding the heat stability of proteins adds a layer of knowledge that can be important in Sports Nutrition.